Org. Biomol. Chem., 2008, 6, 2507-2515.
Towards sequence selective DNA binding: design, synthesis and DNA binding studies of novel bis-porphyrin peptidic nanostructures
Éric Biron and Normand Voyer
Abstract
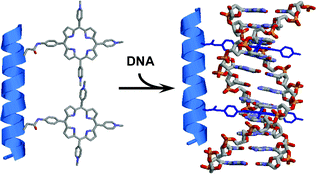
A new series of peptidic nanostructures bearing two intercalating moieties was designed and synthesized to achieve selective recognition of DNA sequences. A cationic porphyrin was attached to a glutamic acid side chain and the latter introduced into a peptidic sequence by standard solid-phase peptide synthesis methodology. Conformation of the hydrosoluble peptidic structures bearing two cationic porphyrins was studied by circular dichroism. Using UV–visible spectroscopy and induced circular dichroism, we demonstrate that the compounds are fully intercalated upon binding to double-stranded DNA and that the compounds exhibit a tremendous preference for GC over AT sequences for intercalation
Graphical abstract:
.