Food hydrocolloids, 2023.
Hydrogel formation from peptides of a ▀-lactoglobulin tryptic hydrolysate: Contribution of self-assembling peptide ▀-Lg f1-8
Mathilde Pimont-Farge, Veronique Perreault, Guillaume Brisson, Franšois Otis, Normand Voyer, Shyam Suwal, Yves Pouliot, Alain Doyen
Abstract
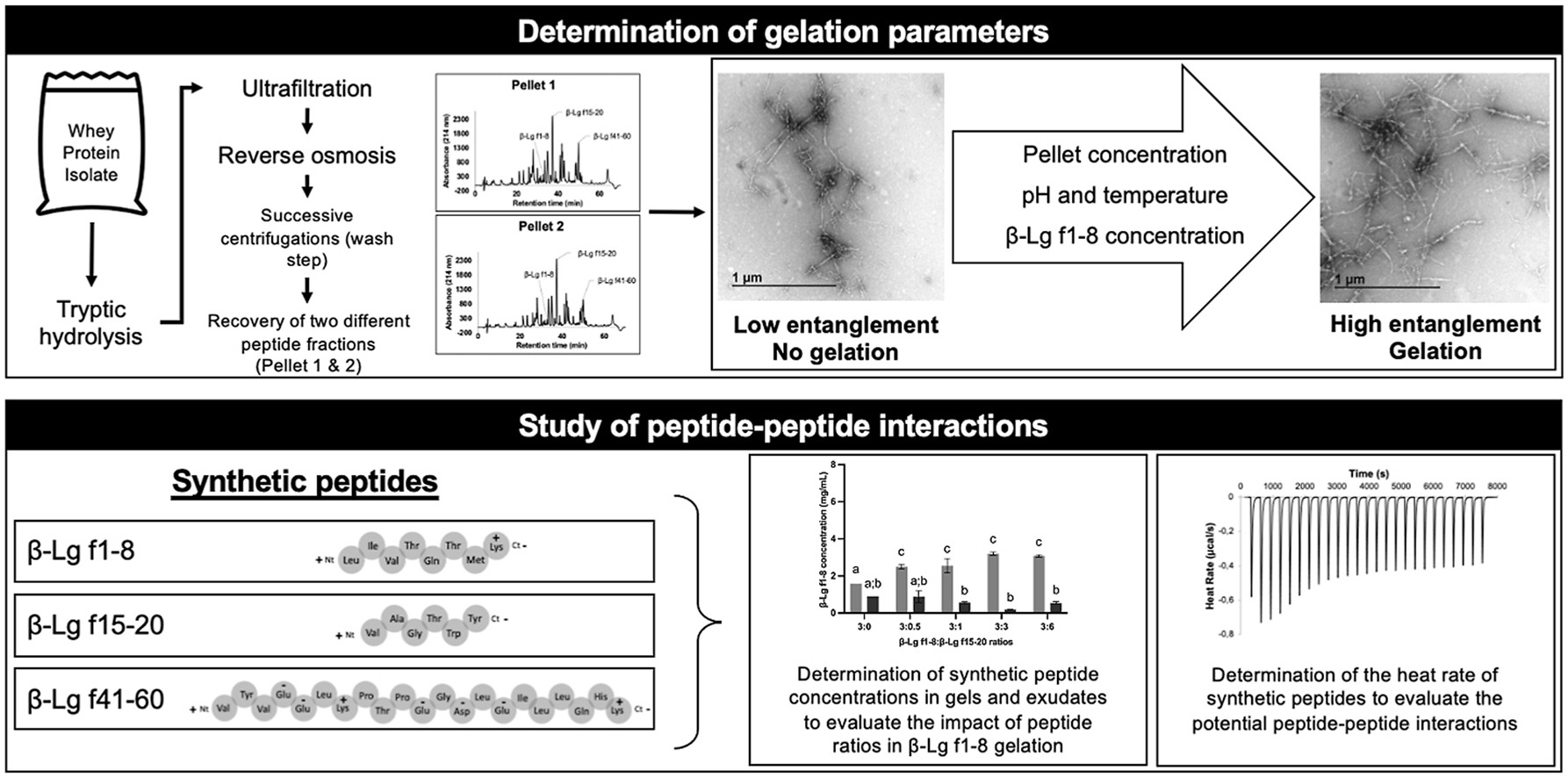
Peptide-based hydrogels, typically mediated by non-covalent interactions, are becoming increasingly attractive due to their many biotechnological applications such as drug and antimicrobial delivery, as well as tissue engineering. In our previous work, we demonstrated that specific peptide fractions from beta-lactoglobulin (▀-Lg) could form hydrogels either by peptide-peptide interaction or through peptide self-assembly of ▀-Lg f1-8, depending on the purity of ▀-Lg f1-8 in peptide fractions. However, mechanisms that govern hydrogel formation were not investigated. Consequently, this study focused on the impact of peptide fraction (pellet) concentration, temperature, pH and ▀-Lg f1-8 concentration on hydrogel formation. Moreover, contribution of peptides ▀-Lg f15-20 and ▀-Lg f41-60 to hydrogel formation was determined. We hypothesized that hydrogel formation results from a balance between hydrogen, hydrophobic and electrostatic interactions during gelation at a concentration of 50 mg/mL, 20 ░C and pH close to 11. The peptide ▀-Lg f1-8 was shown to initiate the gelation by increasing the density of nanofibers in a concentration-dependent manner which promoted peptide-peptide interactions. Finally, interactions between both ▀-Lg f15-20 and ▀-Lg f41-60 peptides and ▀-Lg f1-8 occurred during gel formation negatively impacted the self-assembly process. The proposed mechanism for ▀-Lg f1-8 gelation would facilitate the development of functional soft biomaterials and bioactive encapsulation systems.